what allows the contraction cycle to repeat so that shortening of the sarcomere happens?
Learning Objectives
- Explicate the roles of ATP, cytoskeletal proteins, and motor proteins in cilia, flagella, and muscle in controlling jail cell and organism motility
- Describe the structure of the muscle and explain the role of this structure in muscle contraction and regulation of musculus contraction through the sliding filament model
- Explicate the process of the cross-bridge cycle
- Explicate the process by which the nervous organisation and the sarcoplasmic reticulum regulate muscle contraction
Motor Proteins and Cytoskeletal Tracks
The information beneath was adapted from Khan Academy: The cytoskeleton. All Khan Academy content is bachelor for free at world wide web.khanacademy.org
Unlike plants (and constitute cells), animals (and often private beast cells) are capable of movement. This process requires both a business firm structure to provide leverage as well as a machinery for moving that construction. In multicellular animals, these components are the skeleton and muscles. In unmarried-celled animals and individual cells, these components are often flagella and/or cilia. All of these structures rely on bothmotor proteins and components of thecytoskeleton.
The cytoskeleton(literally, "cell skeleton") is a network of filaments that supports the plasma membrane, gives the jail cell an overall shape, aids in the right positioning of organelles, provides tracks for the transport of vesicles, and (in many cell types) allows the cell to move. In eukaryotes, in that location are 3 types of protein fibers in the cytoskeleton: microfilaments, intermediate filaments, and microtubules. Mirofilaments and microtubles serve as tracks for movement of motor proteins, which use energy in the class of ATP to "walk forth" these cytoskeletal filaments. (Intermediate filaments are not associated with motor proteins.) For the purposes of this grade, we'll talk over microfilaments, microtubules, and their associated motor proteins to describe their roles in cilia, flagella, and musculus:
- Microfilaments (besides chosen actin filaments) take a double helix-similar construction equanimous ofactin protein subunits; microfilaments serve as tracks for the motor proteinmyosin and are involved in many cellular processes that require motion. In this grade, we will focus on their role in musculus cells, where they form organized structures of overlapping filaments called sarcomeres. When the actin and myosin filaments of a sarcomere slide by each other in concert, your muscles contract (much, much more than on this afterward in this page).
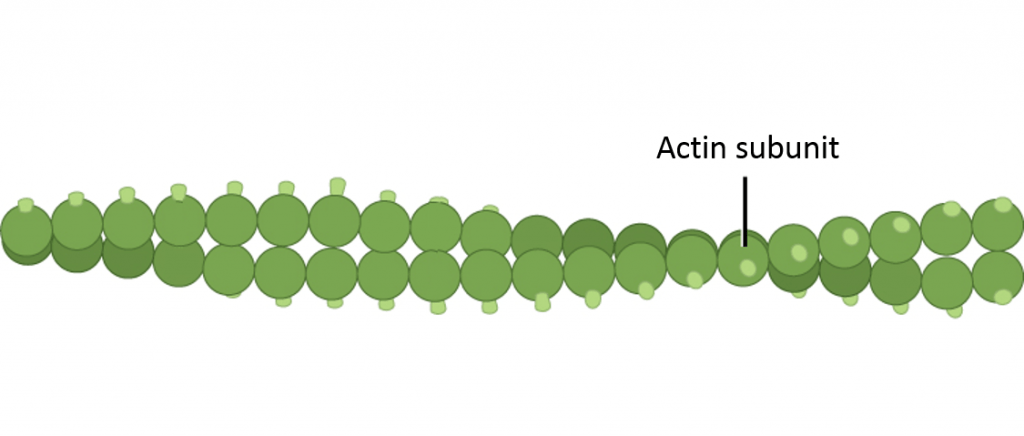
Double helical structure of microfilament, composed of two intertwined strands of actin subunits. Image credit: Modification of work from Khan Academy https://www.khanacademy.org/scientific discipline/biology/structure-of-a-cell/tour-of-organelles/a/the-cytoskeleton, originally modified from OpenStax Biology
- Microtubules are made upwardly of tubulin proteins bundled to form a hollow, straw-like tube that serve as tracks for the motor proteins kinesin anddyenin; microtubules play important roles in both cellular structural integrity and cell movement; in this form we will focus on their roles in cell movement via cilia and flagella.

Microtubules are hollow structures composed of polymerized dimers of tubulin (right image). The left epitome shows the molecular structure of the tube. Image credit: OpenStax Biological science
- Motor proteins apply energy in the form of ATP to "walk" along specific cytoskeletal tracks. They are essential for movement of vesicles and other cargoes within cells, too as for the motility of musculus and cilia/flagella:
- Myosin is associated withactin microfilaments and is required for movement ofmuscle
- Dynein is associated withtubulin microtubules and is required for movement of ciliaandflagella
- Kinesin is associated withtubulin microtubules and is required for movement of vesicles and other intracellular ("within cell") cargoes
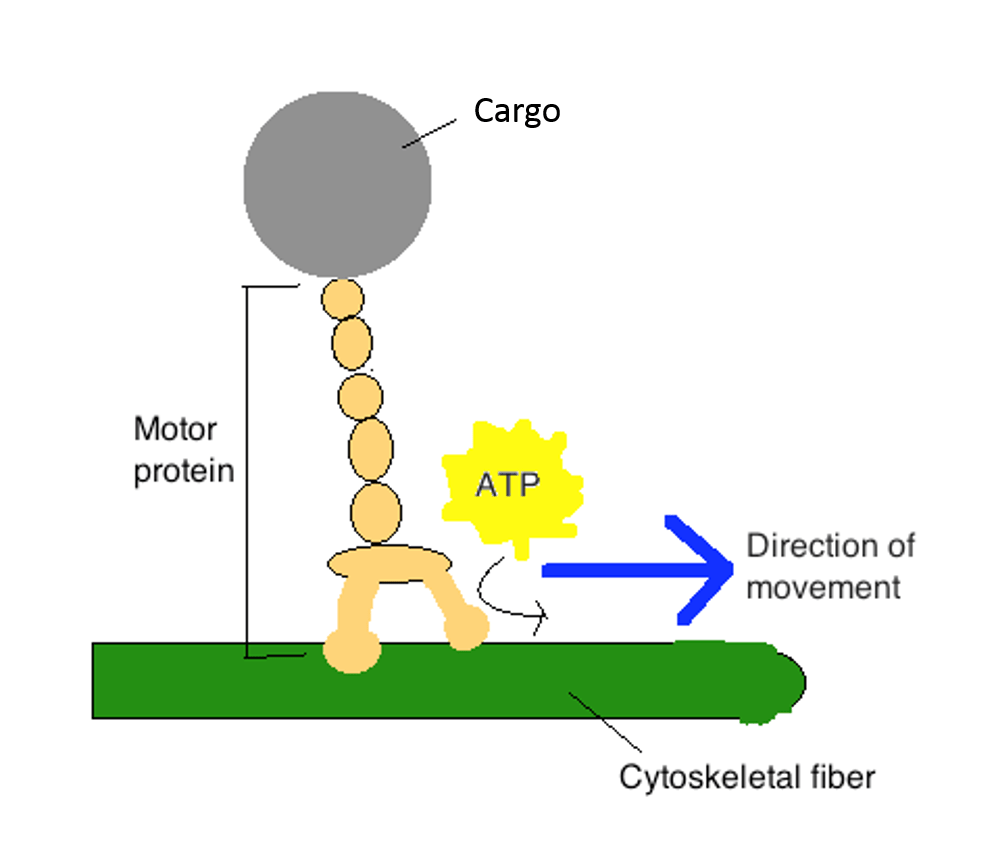
Motor proteins consist of ii heads that "walk" along a cytoskeletal track, and a tail that is connected to cargo or some other structure. Modification of piece of work past Ccl005 (Own work) [CC Past-SA 3.0 (http://creativecommons.org/licenses/by-sa/iii.0)], via Wikimedia Eatables
The video below, an excerpt from "The Inner Life of a Cell" by Cellular Visions and Harvard (http://www.studiodaily.com/2006/07/cellular-visions-the-inner-life-of-a-cell/), shows the motor poly peptide kinesin walking along a microtubule track:
Cilia and Flagella
The information below was adapted from Khan Academy: The cytoskeleton. All Khan Academy content is available for costless at www.khanacademy.org
ATP, dynein motor proteins, and microtubule tracks are essential for motion of eukaryotic cilia and flagella. Flagella (atypical, flagellum) are long, hair-like structures that extend from the cell surface and are used to movement an entire cell, such as a sperm. If a cell has any flagella, it ordinarily has one or just a few. (Note that, while they carry out the aforementioned function, the eukaryotic flagella discussed here has a fundamentally different structure from the prokaryotic flagella.) Motile cilia (singular, cilium) are similar, merely are shorter and usually appear in large numbers on the cell surface. When cells with motile cilia form tissues, the beating helps move materials across the surface of the tissue. For example, the cilia of cells in your upper respiratory system aid move grit and particles out towards your nostrils.
Despite their departure in length and number, flagella and motile cilia share a common structural pattern and mechanism driving motion:
- In about flagella and motile cilia, there are 9 pairs of microtubules arranged in a circle, forth with an additional ii microtubules in the center of the ring. This arrangement is called a nine + 2 array. You can come across the 9 + 2 array in the electron microscopy prototype at left, which shows two flagella in cantankerous-department.
- The individual pairs of microtubules are physically connected to each other via poly peptide bridges. They are also physically anchored to the base of the cilium or flagellum.
- The pairs of microtubules are also physically connected via motor proteins called dyneins that motion along the microtubules, generating a force that causes the flagellum or cilium to beat. The dynein is anchored to one doublet, and "walks along" the side by side doublet; the synchronous move of all dyneins across the entire structure cases the cilium or flagellum to curve.
- The structural connections between the microtubule pairs and the coordination of dynein movement allow the activity of the motors to produce a pattern of regular beating, driving the cell to move in a coordinated fashion.

This transmission electron micrograph of two flagella shows the nine + 2 array of microtubules: nine microtubule doublets environs a single microtubule doublet. Dynein proteins are physically anchored to one doublet in a microtubule band construction and "walk forth" an next doublet, causing the entire construction to bend and beat. Image credits Khan Academy https://world wide web.khanacademy.org/science/biology/structure-of-a-cell/tour-of-organelles/a/the-cytoskeleton; upper panel, "The cytoskeleton: Figure 5," past OpenStax Higher, Biology (CC Past 3.0). Modification of work by Dartmouth Electron Microscope Facility, Dartmouth Higher; calibration-bar information from Matt Russell. Lower panel, modification of "Eukaryotic cilium diagram," by Mariana Ruiz Villareal (public domain).
Muscle Contraction and Locomotion
The information below was adapted from OpenStax Biology 38.iv
ATP, motor motor proteins, and actin microfiliament tracks are essential for contraction of eukaryotic muscle. Muscles allow for motions such as walking, and they also facilitate bodily processes such as respiration and digestion. The vertebrate body contains 3 types of musculus tissue: skeletal muscle, cardiac muscle, and smooth musculus:
- Skeletal muscle tissue forms skeletal muscles, which adhere to bones or skin and control locomotion and any move that tin can be consciously controlled. Considering it can exist controlled by thought, skeletal muscle is also called voluntary muscle. Skeletal muscles are long and cylindrical in advent; when viewed under a microscope, skeletal muscle tissue has a striped or striated appearance. The striations are caused by the regular arrangement of contractile proteins (actin and myosin). Actin is a globular contractile protein that interacts with myosin for muscle contraction. Skeletal musculus also has multiple nuclei nowadays in a unmarried cell.
- Smooth muscle tissue occurs in the walls of hollow organs such as the intestines, breadbasket, and urinary bladder, and around passages such equally the respiratory tract and blood vessels. Smooth muscle has no striations, is not under voluntary control, has only i nucleus per prison cell, is tapered at both ends, and is called involuntary muscle.
- Cardiac muscle tissue is just found in the heart, and cardiac contractions pump claret throughout the torso and maintain blood pressure. Like skeletal muscle, cardiac musculus is striated, but unlike skeletal muscle, cardiac musculus cannot be consciously controlled and is called involuntary muscle. The nervous system can speed upwards or slow down the centre rate, but the heart can also beat without nervous organisation input due to a prepare of cells chosen pacemaker cells that spontaneously initiate cardiac muscle contraction. Information technology has one nucleus per cell, is branched, and is distinguished by the presence of intercalated disks, which enable rapid passage of action potentials from one cardiac muscle jail cell to the next.
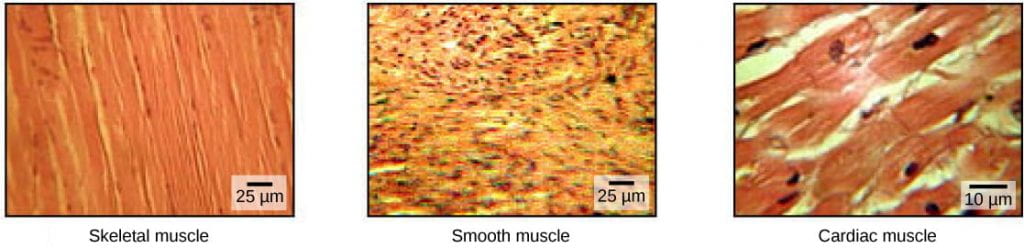
The body contains 3 types of muscle tissue: skeletal musculus, polish musculus, and cardiac musculus, visualized here using calorie-free microscopy. Polish muscle cells are short, tapered at each end, and have but one plump nucleus in each. Cardiac muscle cells are branched and striated, but curt. The cytoplasm may branch, and they take i nucleus in the middle of the cell. (credit: OpenStax Biology, modification of work past NCI, NIH; scale-bar data from Matt Russell)
Muscles are composed of structures that enable wrinkle to promote organsimal move. Each skeletal muscle fiber is a single skeletal muscle cell. These cells are incredibly large, with diameters of upwards to 100 µm and lengths of up to 30 cm. Within each muscle cobweb are myofibrils:long cylindrical structures that prevarication parallel to the muscle cobweb. Myofibrils run the entire length of the musculus fiber, and considering they are only approximately 1.2 µm in diameter, hundreds to thousands tin can exist found inside ane musculus fiber. Each myofibril contains repeating units chosensarcomeres, which are the actual functional units that crusade contraction of a muscle (the "contractile units"). Sarcomeres give muscle its striated or banded appearance, due to the alternating bands of actin and myosin that let sarcomeres to contract .
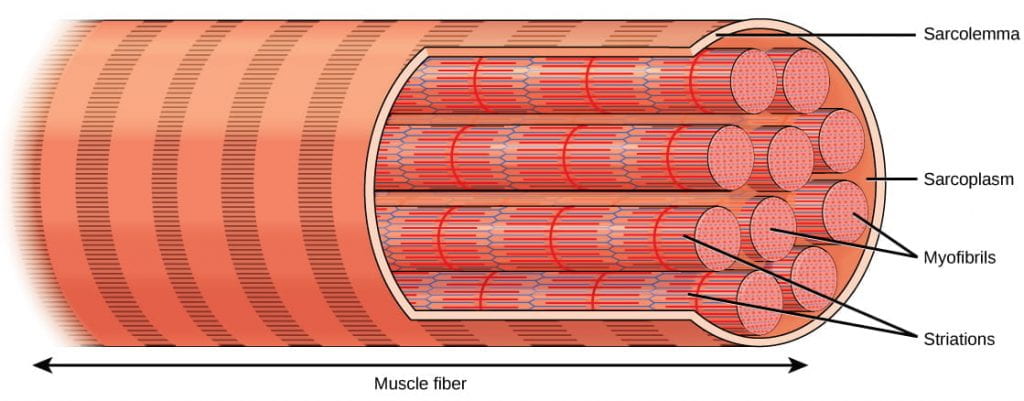
A skeletal muscle cell (musculus fiber) is surrounded by a plasma membrane called the sarcolemma with a cytoplasm called the sarcoplasm. A muscle fiber is equanimous of many myofibrils, packaged into orderly units. Image credit: OpenStax Biology.
Each sarcomere contains a thick (night) filament ofmyosin, and a thin (light) filament ofactin. The actin filaments are physically anchored to structures at the terminate of each sarcomere, chosenZ discs or Z lines. The center of the myosin filament is marked past a structure chosen theM line. One sarcomere is the infinite between two consecutive Z discs. A myofibril is composed of many sarcomeres running forth its length, and as the sarcomeres individually contract, the myofibrils and muscle cells shorten. Importantly,the actin and myosin fibers stay the same length by sliding past each otheras the prison cell itself shortens.
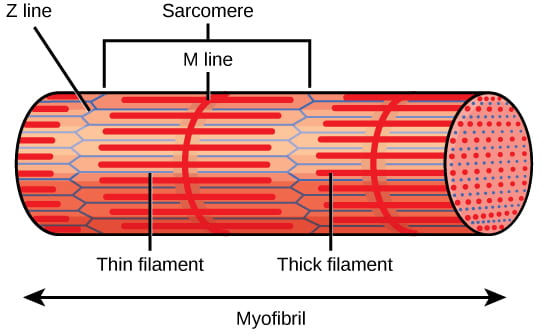
A sarcomere is the region from i Z line to the next Z line. Many sarcomeres are present in a myofibril, resulting in the striation pattern feature of skeletal muscle. Prototype credit: OpenStax Biological science.
The protein components of the sarcomere include actin, myosin, tropomyosin, and troponin:
- Thick filaments are composed of the protein myosin. The tail of a myosin molecule connects with other myosin molecules to form the central region of a thick filament near the M line, whereas the heads marshal on either side of the thick filament where the thin filaments overlap.
- The principal component of thin filaments is the actin poly peptide. Actin filaments are attached at the Z disc and extend towarrd the M line, overlapping with the myosin heads of the thick filament
- Ii other components of the thin filament are tropomyosin and troponin. Actin has binding sites for myosin attachment, simply strands of tropomyosin block the binding sites and foreclose actin-myosin interactions when the muscles are at rest. Tropomyosin is regulated by troponin, which is regulated by calcium (Ca2+) ions. The bounden of Ca2+ to troponin causes the troponin-tropomyosin complex to motion away from the myosin binding sites on the actin filament, allowing myosin to bind to actin to initiate muscle contraction (more on this later).
The video beneath describes the organization of musculus fibers:
How does the construction of muscle allow for muscle contraction? For a musculus cell to contract, the sarcomere must shorten. However, thick and sparse filaments (the components of sarcomeres) do not shorten. Instead, they slide by one another, causing the sarcomere to shorten while the filaments remain the same length. The sliding filament model of musculus contraction explains the movement of the bands on the sarcomere at different degrees of musculus contraction and relaxation. The mechanism of wrinkle is the binding of myosin to actin, forming cross-bridges that generate filament motion. Thus the sliding filament model is accomplished by the cross-bridge bike of actin-myosin binding.
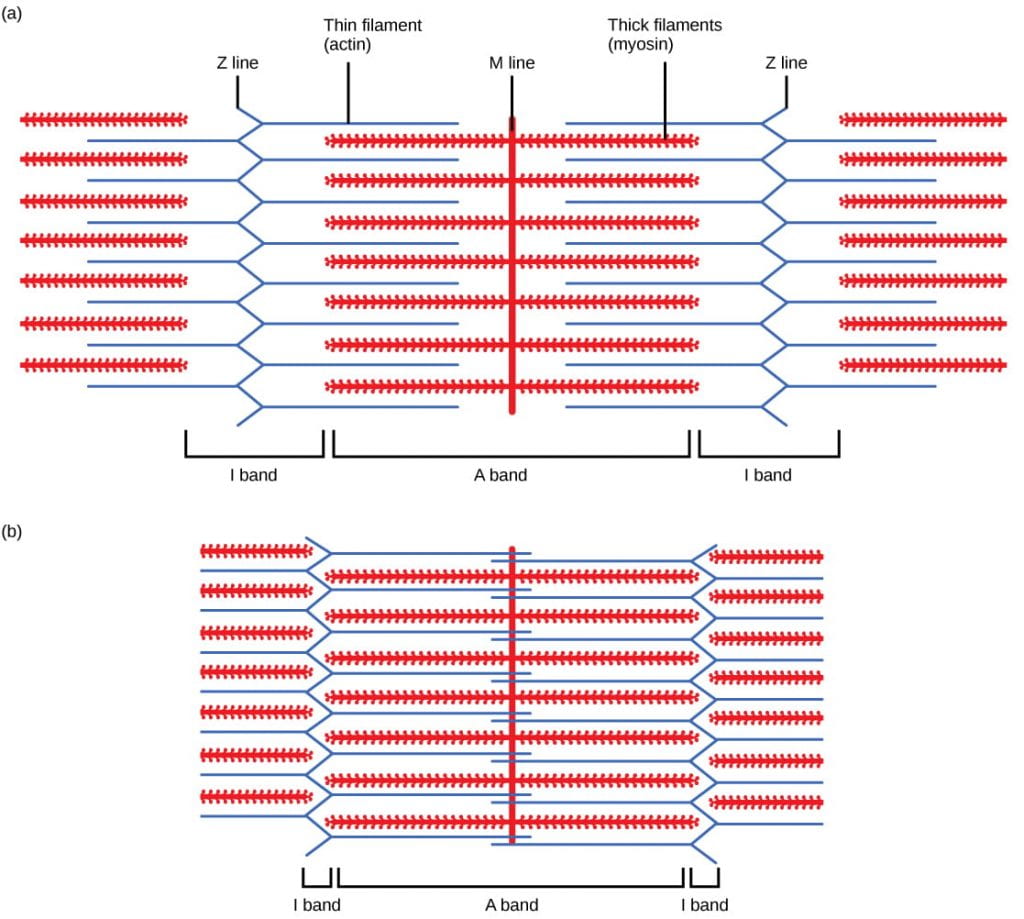
When (a) a sarcomere (b) contracts, the Z lines motility closer together and the I ring gets smaller. The A band stays the aforementioned width and, at full contraction, the thin filaments overlap. Paradigm credit: OpenStax Biology.
When a sarcomere shortens, the altitude betwixt the Z discs is reduced even though the filaments within the sarcomere stay the same length. This is considering thin filaments are pulled by the thick filaments toward the center of the sarcomere until the Z discs arroyo the thick filaments. The zone of overlap, in which thin filaments and thick filaments occupy the same surface area, increases as the thin filaments move inward, every bit illustrated below:

Blithe molecular view of muscle showing actin (ruddy) and myosin (pink) sliding filament contracting.By hamish darby – Own work, Public Domain, https://commons.wikimedia.org/westward/index.php?curid=18774740
How does this process work? The motion of musculus shortening occurs as myosin heads bind to actin and pull the actin in. This activeness requires free energy, which is provided by ATP. Myosin binds to actin at a binding site on the globular actin poly peptide. Myosin has some other binding site for ATP at which enzymatic activity hydrolyzes ATP to ADP, releasing an inorganic phosphate molecule and energy. The procedure works in a wheel (called the "cantankerous bridge cycle") like this:
- At the start of a cycle, myosin binds to ATP, simply it is not yet leap to actin.
- Myosin then hydrolyzes ATP into ADP and inorganic phosphate, and the myosin remains bound to both of these molecules. In this land, information technology can and then adhere to a myosin-binding site on the actin thin filament if the binding sites are available (if non, the myosin will remain in this state until the binding sites become available).
- After binding to actin, the myosin protein releases the inorganic phosphate (but stays jump to ADP); the release of inorganic phosphate causes the myosin caput to ratchet in what is called "the ability stroke,"pulling the actin thin filament toward the One thousand line and causing muscle contraction.
- After the power stroke is completed, the myosin protein releases ADP. In this country, it remains stuck to the actin filament until information technology binds another ATP molecule. Considering ATP is required for myosin to be able to release the actin, depletion of ATP due to muscle fatigue will cause muscles to remain locked in a contracted country; this is thought to be ane of several sources of cramping after practice.
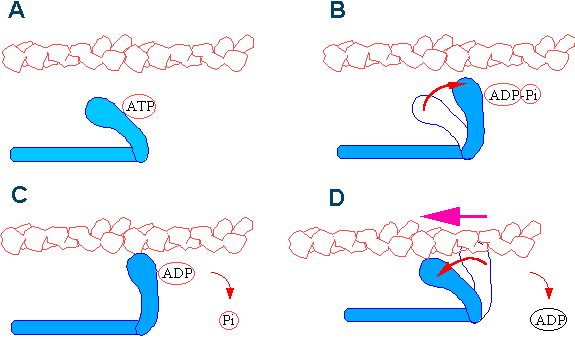
The Myosin Cantankerous-bridge Cycle. A. ATP binding to a scissure at the "back" of the head causes a conformation which cannot demark actin. B. Equally the ATP is hydrolysed, the head swings back about 5nm to the "cocked" position the ADP and Pi remain bound. C+D. The force generating stages. When the Pi leaves the myosin, the head binds the actin and the "power stroke" is released as the caput bind actin. ADP is released to continue the cycle. At this stage the head in bound to actin in the "rigor" or tightly leap state. Each wrinkle cycle causes actin to move relative to myosin. Image credit: Sutherland Maciver, http://world wide web.bms.ed.ac.uk/research/others/smaciver/Myosin%20II.htm, used with permission.
The video below shows the cross bridge bicycle of muscle contraction:
How is muscle activity regulated? Subsequently all, unless an brute has but utilized a massive amount of energy (perhaps running to catch nutrient – or escape from a hungry predator!), in that location is e'er ATP available in the muscle, however our muscles aren't permanently contracting. The regulation of muscle contraction is accomplished via the troponin-tropomyosin complex (and the nervous system, which we'll discuss in item on the next folio). As mentioned above, troponin and tropomyosin determine whether myosin can bind to actin or not:
- When a muscle is in a resting state, actin and myosin are separated. To keep actin from bounden to the active site on myosin, tropomyosin blocks myosin bounden sites on actin molecules, preventing cross-bridge formation and preventing contraction in a muscle without nervous input. Troponin binds to and regulates tropomyosin.
- To enable a muscle contraction, tropomyosin must change conformation, uncovering the myosin-binding site on an actin molecule and allowing cross-span formation. This tin can only happen in the presence of calcium, which is kept at extremely low concentrations in the sarcoplasm (muscle prison cell cytoplasm). If present, calcium ions demark to troponin, causing conformational changes in troponin that let tropomyosin to move abroad from the myosin binding sites on actin. Once the tropomyosin is removed, a cross-bridge tin can class between actin and myosin, triggering contraction. Cross-span cycling continues until Ca2+ ions and ATP are no longer available and tropomyosin over again covers the binding sites on actin.
What regulates the presence or absence of calcium? The availability of calcium is regulated by the nervous system. Calcium is stored in a the endoplasmic reticulum, which is chosen thesarcoplasmic reticulum (SR)in muscle cells. The efferent neurons which control a musculus are synapsed with the muscle in a structure called theneuromuscular junction orneuromuscular synapse:
- When an activity potential propagates down the axon of the efferent neuron, the neurotransmitteracetylcholine (ACh) is released by the neuron into the synaptic cleft of the neuromuscular junction.
- ACh binds to ACh receptors on the musculus cell, which depolarizes the muscle cell and initiates an action potential in the musculus using exactly the aforementioned chemical science as an action potential in a neuron (influx of sodium ions, efflux of potassium ions)
- The depolarization of the musculus jail cell and so spreads through structures called T-tubules, which carry the action potential into thesarcoplasmic reticulum(SR).
- Depolarization of the SR causes the SR to release calcium into the muscle cytoplasm.
- The sudden presence of calcium in the cytoplasm causes the tropoin-tropomyosin circuitous to move abroad from blocking the myosin bounden sites on the actin thin filament, allowing musculus contraction to occur.
- After the terminate of the activeness potential, the muscle jail cell returns to its membrane resting potential and calcium is apace pumped back into the SR, ending muscle contraction and allowing the muscle to relax (as long equally ATP is nowadays to cause myosin to release the actin).
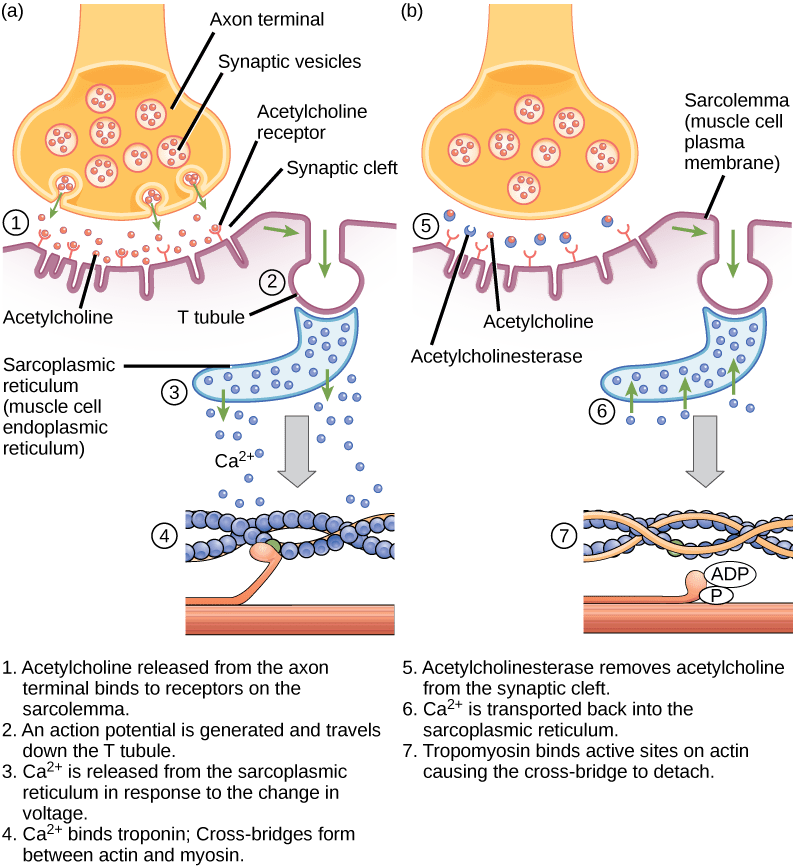
This diagram shows the role of the nervous arrangement in regulating skeletal muscle contraction. The sarcoplasmic reticulum is a specialized endoplasmic reticulum found in musculus cells. In that location are iv steps in the beginning of a muscle contraction. Epitome credit: OpenStax Biology.
This video describes how neuronal signaling initiates musculus contraction (also called excitation-contraction coupling):
And this video puts it all together to describe how musculus contraction works to facilitate locomotion:
Source: https://organismalbio.biosci.gatech.edu/chemical-and-electrical-signals/effectors-and-movement/
0 Response to "what allows the contraction cycle to repeat so that shortening of the sarcomere happens?"
Post a Comment